LC & LC-MS compliant with In Vitro Diagnostic Regulation (IVDR)
Shimadzu offers Liquid Chromatography and Mass Spectrometry medical devices.

What is the New EU In Vitro Diagnostic Regulation (IVDR)?
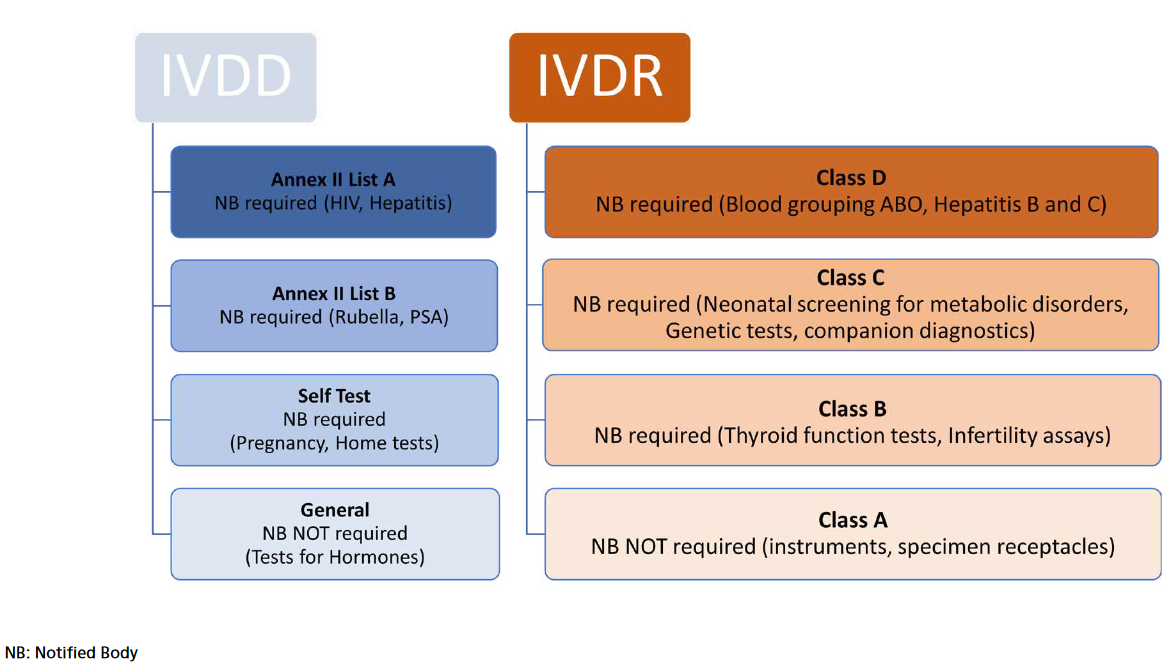
Figure 1. IVDR risk-based classification
New In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746 was published in EU Official Journal in May 2017 in order to ensure a high level of safety and health whilst supporting innovation. It applies from 26 May 2022 and replaced the previous IVD Directive (98/79/EC). This regulation set rules for in vitro diagnostic devices such as reagent, calibrator, kit, instrument, and software intended by the manufacturer to be used for diagnostic purposes. IVDR retains some concepts of IVD Directive such as CE marking or essential requirements (under IVDR called “general safety and performance
requirements”). As in the past, the manufacturers have to demonstrate that they and their devices meet the requirements through conformity assessment procedure. Different from IVD Directive, however, IVDR introduced the riskbased classification (Figure 1). Under IVDR devices are classified in four: The devices with highest risks are classified in class D. The class A devices have low risks and include products for general laboratory use, accessories which possess no critical characteristics.
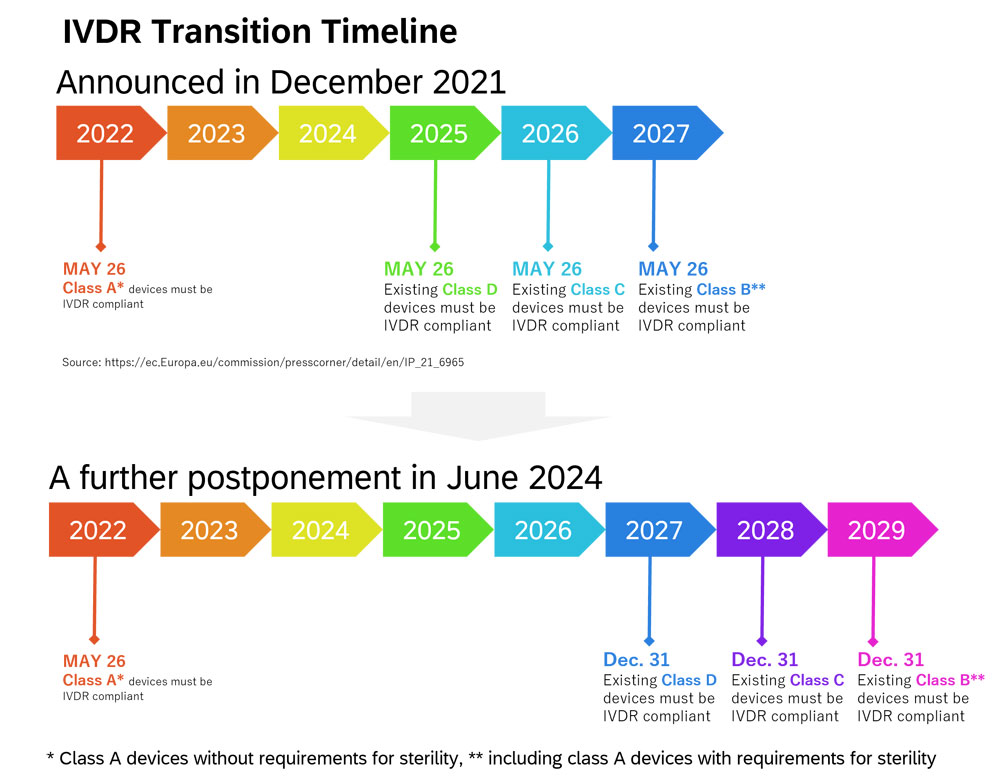
Figure 2. IVDR Transition Timeline
As shown Figure 2, self-declaration of conformity by the manufacturer which meet the requirements of IVDR is necessary to place the class A devices* on the EU market from 26 May 2022. Class B**, C, and D devices need the certification from the accredited Notified Body (NB) to be placed on the EU market. While only 8 % of devices required NB certification under the IVD Directive, 78 % of devices require NB certification under IVDR.1 Despite the fact, the number of NBs accredited in the EU is small.2 For that reason, in December 2021, the transition period has been postponed depending on the class (class B** until 26 May 2027, class C until 26 May 2026, and class D until 26 May 2025). The European Commission published a further amendment in June 20243 , and the transition period of class B** devices will be postponed until 31 December 2029, class C until 31 December 2028 and class D until 31 December 2027.
Shimadzu's CL Series
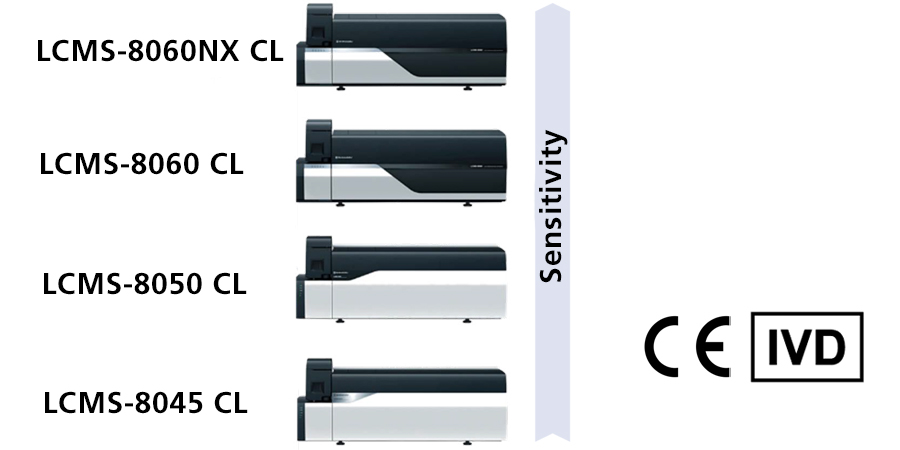
If a Research Use Only (RUO) HPLC or LC-MS/MS is used for diagnostic purposes, it may be converted to in-house device in accordance with Article 5(5) of IVDR. However, the work required to meet the requirements of Article 5(5) is enormous. Therefore, we recommend the introduction of IVDR class A products for HPLC and LC-MS/MS to reduce the workload. Shimadzu has already launched IVDR Class A devices for HPLC (LC-40 CL) and LC-MS/MS (LCMS-8045/8050/8060/8060NX CL) in 2022. HPLC IVDR class A is available without MS. Each unit (pump, autosampler, oven, valve, and detector (UV)) is IVDR Class A, so the combination can be customized. For LC-MS/MS, series can be selected according to required sensitivity.
Shimadzu also launched an IVDR Class A device, the CLAM-2040 CL, a fully automated sample preparation module for Shimadzu LC-MS/MS that enables a seamless workflow from laborious pre-treatment to fully automated LC-MS/MS. The CLAM-2040 CL is not only seamless, but also flexible. Depending on the application, you can choose an IVDR class A compliant LC-MS/MS to be combined with the CLAM-2040 CL. The design also allows connection to LIS (Laboratory Information System) and LAS (Laboratory Automation System), enabling a wide range of tests and workflow improvements using LC-MS/MS. This IVD devices, in combination with commercially available IVD kit, is expected to relieve the user from the enormous amount of work necessary to meet the requirements of Article 5(5) of IVDR. Only the validation by combination is still required.
CL Series Liquid Chromatograph and Mass Spectrometry Listed Devices (for EU CE-IVD):
| Main Module | P/N | ||
|---|---|---|---|
| LC | Communications | CBM-40 CL | 228-45012-55 |
| Nexera CL Liquid Chromatograph | LC-40D X3 CL | 228-65076-55 | |
| LC-40D XR CL | 228-65000-55 | ||
| LC-40B X3 CL | 228-65078-55 | ||
| Degassing Unit | DGU-405 CL | 228-65019-55 | |
| Nexera CL Autosampler | SIL-40C X3 CL | 228-65120-55 | |
| SIL-40C XR CL | 228-65103-55 | ||
| Nexera CL Column Oven | CTO-40C CL | 228-65202-55 | |
| Valve Unit | FCV-S CL | 228-65600-55 | |
| Nexera CL UV Detector | SPD-40 CL | 228-65312-55 | |
| LCMS | Triple Quad LC-MS/MS | LCMS-8045 CL | 225-31400-55 |
| 225-31800-55 | |||
| LCMS-8050 CL | 225-19600-55 | ||
| 225-19610-55 | |||
| LCMS-8060 CL | 225-27800-55 | ||
| 225-27810-55 | |||
| LCMS-8060NX CL | 225-41600-55 | ||
| 225-41690-55 | |||
| Automation | CLAM automation module | CLAM-2040 CL | 241-18700-55 |
| Software | Software LC&LCMS | LabSolutions CL Ver. 1.40 (DVD) | 225-45811-55 |
| Software LCMS | LabSolutions Insight CL Ver. 1.1 (DVD) | 225-45812-55 | |
| LabSolutions CL/LabSolutions Insight CL license ASSY | 225-45813-55 | ||
| Software LC | LabSolutions CL Single LC (License) | 223-63386-55 | |
| LabSolutions CL Add LC (License) | 223-63387-55 |
NB: These instruments are only available in the target market where the conformity assessment procedure of the product (CE IVDR) is accepted.
These instruments are designed/developed, manufactured, installed and repaired under a certified Quality Management System according EN ISO 13485:2016 for medical devices and related services.
References
- MedTech Europe Survey Report, 8 September 2021.p.6-7.
medtech-europe-survey-report-analysing-the-availability-of-in-vitro-diagnostic-medical-devices-ivds-in-may-2022-when-the-new-eu-ivd-regulation-applies-8-september-2021.pdf (medtecheurope.org) - As of 2 May 2025, only 17 Notified Bodies exist.
EUROPA – European Commission – Growth – Regulatory policy - SMCS - Publications Office (europa.eu)
Regulation (EU) 2024/1860 of the European Parliament and of the Council of 13 June 2024 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards a gradual roll-out of Eudamed, the obligation to inform in case of interruption or discontinuation of supply, and transitional provisions for certain in vitro diagnostic medical devicesText with EEA relevance. (europa.eu) - MDCG 2023-1 Guidance on the health institution exemption under Article 5(5) of Regulation (EU) 2017/745 and Regulation (EU) 2017/746, January 2023, p.3.
mdcg_2023-1_en.pdf (europa.eu) - mdcg_2023-1_en.pdf (europa.eu)
- HYPERLINK EUR-Lex - 52024PC0043 - EN - EUR-Lex (europa.eu)
- MDCG 2022-8 Regulation (EU) 2017/746 – application of IVDR requirements to ‘legacy devices’ and to devices placed on the market prior to 26 May 2022 in accordance with Directive 98/79/EC
IVD legacy devices (europa.eu).